Medical device testing and development
There are several aspects to consider when testing medical devices:
- Powering the device under test (DUT)
- Battery life and power consumption
- Power integrity
- Signal integrity
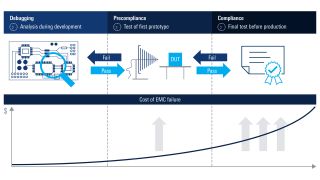
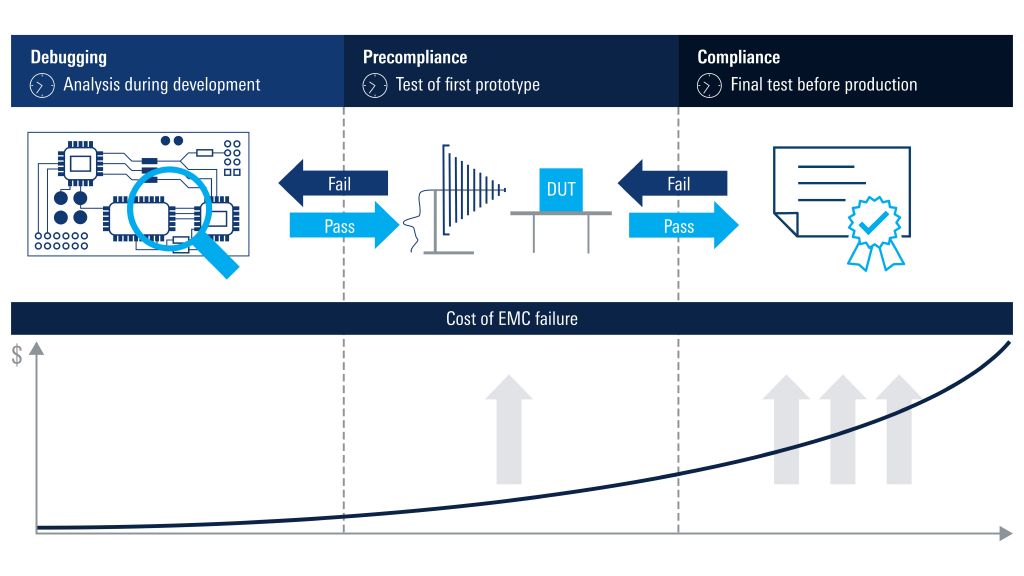
- Precompliance EMI debugging
- Measuring small sensor signals
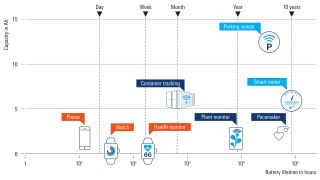
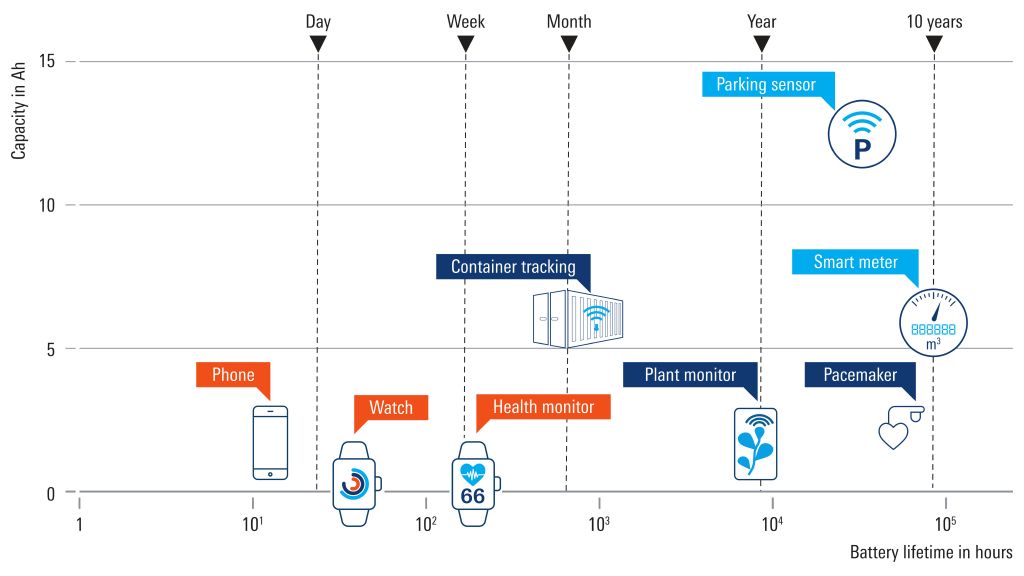
Battery life is crucial for many medical applications, including:
- Pacemakers
- Prosthetics
- Neurostimulation
- Respiratory assistance
- Defibrillators
A battery simulator is necessary to evaluate how long a device can be powered and functionality guaranteed. There are two main testing aspects, and it is possible to optimize for both:
1. Functionality based on battery stages: A new battery has zero resistance, but this increases with age and affects the circuitry. A battery’s behavior also changes with its discharge status.
2. Power consumption: The expected power consumption of a device must be established to determine battery lifetime.
Power supplies and source measure units (SMU) are often used for battery simulation. These instruments can emulate the real output behavior of a battery over time. However, unlike a battery, a power supply draws significantly more current when it becomes active, leading to a voltage drop. Fast voltage regulation is crucial to reduce the effect of this phenomenon and more accurately simulate real battery behavior.
As medical devices grow more compact and streamlined, supplying clean and stable supply voltages becomes an increasing problem. Testing electronic designs for power integrity necessitates extremely sensitive, accurate measurements. Ripple, noise and load step response measurements on integrated circuits, such as ASICs, DDR memories and FPGAs, require:
- Probes that can measure in the single-digit millivolt range
- Measurements of very small disturbances at relatively high DC offset levels